Staff profile
Professor Colin Bain
Pro-Vice-Chancellor (Research)

| Affiliation | Telephone |
|---|---|
| Pro-Vice-Chancellor (Research) in the Department of Chemistry | +44 (0) 191 33 42138 |
| Pro-Vice-Chancellor (Research) in the Vice-Chancellor's Office |
Biography
Research Interests
Chemistry of Surfaces and Interfaces
The research in my group can be loosely characterised as 'wet' surface chemistry. We are motivated by two general questions: What is the relationship between the microscopic structure of a thin film and the molecular structure of its constituent molecules? How do the microscopic properties of an interface determine the macroscopic behaviour of a system? Increasingly we are interested in kinetic processes on timescales from microseconds to seconds and in multi-component systems where interactions between different species lead to unexpected behaviour. Our focus is on fundamental physical chemistry, but the systems we study have potential applications in areas such as lubrication, detergency, printing, manufacturing engineering and oil recovery. Systems are chosen to be sufficiently complex to capture the essential behaviour of real applications, yet simple enough to permit a determination of structure and dynamics at an interface.

We use a wide range of experimental techniques, including evanescent wave Raman scattering, external reflection infrared spectroscopy, sum-frequency spectroscopy, neutron reflection, ellipsometry, tensiometry, optical tweezers and confocal microscopy. Flow cells, overflowing cylinders (right) and high-speed liquid jets are used to provide controlled hydrodynamics. The development of new methodology plays an important part in our research
Surfactants, lipids and polymers
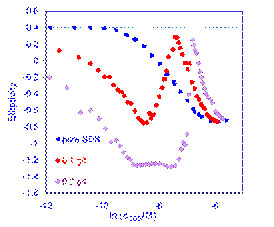
Interactions between species in multi-component systems give rich and unexpected equilibrium and - especially - dynamic behaviour. The graph on the right shows the adsorption of a mixture of the anionic surfactant SDS and a cationic polymer at the expanding surface of an overflowing cylinder, measured by ellipsometry: the lower the reading, the more material is adsorbed at the surface. The complex behaviour arises from interactions between the polymer and surfactant aggregates that first give rise to surface-active complexes and then aggregates that diffuse too slowly to adsorb on the sub-second timescale of the overflowing cylinder.
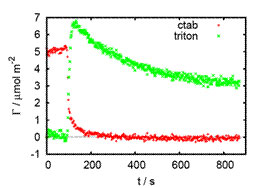
Evanescent wave Raman scattering is a surprisingly sensitive technique for studying mixtures of surfactants or phospholipids at the solid-liquid interface. The plot on the left shows the displacement of the cationic surfactant CTAB from a silica surface by the non-ionic surfactant TX-100. A wall-jet cell and chemometric methods of data analysis permit quantitative measurements of kinetics in mixtures on 1-second timescales.
Liquid jets – micelles, Marangoni and manufacturing
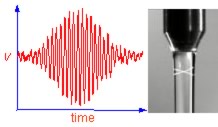
Liquid jets provide a way of studying freshly created surfaces on millisecond and sub-millisecond timescales. Little is known about the adsorption kinetics of surfactants on such short timescales. One surprising observation is that surfactant micelles can adsorb directly to the nascent surface of a liquid jet. Near the jet surface, the solution can be very far from equilibrium with enormous consequences on the kinetics of breakdown of micelles. If surfactant adsorption is not uniform everywhere, surface tension gradients arise and cause flows in the bulk liquid adjacent to the surface. These Marangoni effects are important in stabilising foams, in spreading of droplets on solid surfaces, in the coalescence of droplets and in the breakup of jets. We are applying our understanding of liquid surfaces on short time scales to inkjet printing and to the manufacture of pharmaceuticals by liquid dosing technology.
Molecular tribology

To understand how lubricants work on a molecular level, we need ways of looking into the contact between two hard solids under pressures of thousands of atmospheres and at shear rates >105 s-1. Sum-frequency spectroscopy and Raman scattering have been exploited for this purpose: a circular contact is formed between a hemispherical prism and a sphere and lasers are focused into the small area of contact (the dark region in the centre of the Newton rings, left). Vibrational spectroscopy can tell us which organic molecules are in the contact and how they respond to pressure and shear.
Optical sculpting and binding

Light is an amazing tool for controlling matter on the micro and nanoscale. The scattering of light can induce attractive and repulsive interactions between particles leading the spontaneous organisation of sub-micron polymer spheres into organised arrays (such as the 640-nm diameter PVP spheres on the right).
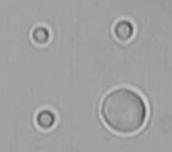
Droplets of oil in water can be picked up and moved with tightly focussed laser beams (optical tweezers) but also deformed into other shapes, such as triangles or squares. When an emulsion droplet is divided into two smaller droplets, they remain connected by a thread of oil that is stable and can be used as a channel to pump liquid from one droplet to the other. The picture on the left shows a 'mother' drop of hexane connected by invisible oil threads to three smaller 'daughter' droplets about a micron in diameter.
References
- C. D. Bain: "Sum-Frequency Vibrational Spectroscopy of the Solid-Liquid Interface" J. Chem. Soc. Faraday Transactions, 1995, 91, 1281.
- Q. Lei, and C. D. Bain: "Surfactant-Induced Surface Freezing at the Alkane-Water Interface" Phys. Rev. Lett., 2004, 92, 176103.
- C. Lee and C. D. Bain: "Raman Spectroscopy of Planar Supported Lipid Bilayers" Biochim. Biophys. Acta, 2005, 1711, 50.
- D. M. Colegate and C. D. Bain: "Adsorption Kinetics in Micellar Solutions of Nonionic Surfactants" Phys. Rev. Lett. 2005, 95, 198302
- C. D. Mellor and C. D. Bain: "Array Formation in Evanescent Waves" ChemPhysChem, 2006, 7, 329.
- A. D. Ward, M. G. Berry, C. M. Mellor and C. D. Bain: "Optical Sculpture: Controlled Deformation of Emulsion Droplets with Ultralow Interfacial Tensions using Optical Tweezers" Chem. Comm., 2006, 4515.
- C. D. Bain: "The Overflowing Cylinder Sixty Years on" Adv. Colloid Interface Sci. 2008, 144, 4.
- E. C. Tyrode, M. W. Rutland and C. D. Bain: "Adsorption of CTAB on Hydrophilic Silica Studied by Linear and Nonlinear Optical Spectroscopy" J. Am. Chem. Soc. 2008, 130, 17434.
Research interests
- Colloid and Interface Science
- Spectroscopy of Surfaces
- Optical Trapping and Binding
Esteem Indicators
- 2005: International awards and named lectures: Lectureship Award of the Chemical Society of Japan, 2005McBain Lecture, National Chemical Laboratory, Pune, India, 2005
Publications
Conference Paper
- An optical platform for the production, trapping, manipulation and visualization of ultra-low interfacial tension emulsion dropletsHargreaves, A. L., Kirby, A. K., Bain, C. D., Love, G. D., Bolognesi, G., Ces, O., Neil, M., & Ward, A. D. (2013). An optical platform for the production, trapping, manipulation and visualization of ultra-low interfacial tension emulsion droplets (K. Dholakia & G. C. Spalding, Eds.). SPIE. https://doi.org/10.1117/12.2027105
- Interaction of peptides and proteins with planar supported lipid bilayers studied by Raman scatteringBain, C., Lee, C., Smith, R., & Wacklin, H. (2006). Interaction of peptides and proteins with planar supported lipid bilayers studied by Raman scattering. In Abstracts of Papers of the American Chemical Society.
- In situ vibrational spectroscopy of thin organic films confined at the solid-solid interfaceWinget, S., Bain, C., & Beattie, D. (2006). In situ vibrational spectroscopy of thin organic films confined at the solid-solid interface. In Abstracts of Papers of the American Chemical Society.
Journal Article
- Evaporation of alcohol droplets on surfaces in moist airYang, L., Pahlavan, A. A., Stone, H. A., & Bain, C. D. (2023). Evaporation of alcohol droplets on surfaces in moist air. Proceedings of the National Academy of Sciences, 120(38), Article e2302653120. https://doi.org/10.1073/pnas.2302653120
- In Situ Self‐Assembly of Nanoscale Particles into Macroscale Ordered Monolayers with Enhanced Memory PerformanceLi, W., Sun, K., Yang, L., Mao, X., Deng, S., Jiang, H., Gu, P., Cao, B., Li, W., Yi, M., Bain, C. D., Deng, R., & Zhu, J. (2023). In Situ Self‐Assembly of Nanoscale Particles into Macroscale Ordered Monolayers with Enhanced Memory Performance. Small, 29(11), Article 2207468. https://doi.org/10.1002/smll.202207468
- Comparative Study of Lipid- and Polymer-Supported Membranes Obtained by Vesicle FusionGoodband, R. J., Bain, C. D., & Staykova, M. (2022). Comparative Study of Lipid- and Polymer-Supported Membranes Obtained by Vesicle Fusion. Langmuir, 38(18), 5674-5681. https://doi.org/10.1021/acs.langmuir.2c00266
- Evaporation of a thin droplet in a shallow well: theory and experimentD’Ambrosio, H., Colosimo, T., Duffy, B., Wilson, S., Yang, L., Bain, C., & Walker, D. (2021). Evaporation of a thin droplet in a shallow well: theory and experiment. Journal of Fluid Mechanics, 927, Article A43. https://doi.org/10.1017/jfm.2021.772
- Evaporation of Binary-Mixture Liquid Droplets: The Formation of Picoliter Pancakelike ShapesPahlavan, A. A., Yang, L., Bain, C. D., & Stone, H. A. (2021). Evaporation of Binary-Mixture Liquid Droplets: The Formation of Picoliter Pancakelike Shapes. Physical Review Letters, 127(2), Article 024501. https://doi.org/10.1103/physrevlett.127.024501
- Wetting and Drying of Aqueous Droplets Containing Nonionic Surfactants CnEmShi, J., Yang, L., & Bain, C. D. (2021). Wetting and Drying of Aqueous Droplets Containing Nonionic Surfactants CnEm. Langmuir, 37(14), 4091-4101. https://doi.org/10.1021/acs.langmuir.0c03479
- A general ink formulation of 2d crystals for wafer-scale inkjet printingHu, G., Yang, L., Yang, Z., Wang, Y., Jin, X., Dai, J., Wu, Q., Liu, S., Zhu, X., Wang, X., Wu, T., Howe, R. C., Albrow-Owen, T., Ng, L. W., Yang, Q., Occhipinti, L. G., Woodward, R. I., Kelleher, E. J., Sun, Z., … Hasan, T. (2020). A general ink formulation of 2d crystals for wafer-scale inkjet printing. Science Advances, 6(33), Article eaba5029. https://doi.org/10.1126/sciadv.aba5029
- In Situ Fabrication of Polymeric Microcapsules by Ink-Jet Printing of EmulsionsDeng, R., Wang, Y., Yang, L., & Bain, C. D. (2019). In Situ Fabrication of Polymeric Microcapsules by Ink-Jet Printing of Emulsions. ACS Applied Materials and Interfaces, 11(43), 40652-40661. https://doi.org/10.1021/acsami.9b14417
- Fabrication of monolayers of uniform polymeric particles by inkjet printing of monodisperse emulsions produced by microfluidicsWang, Y., Deng, R., Yang, L., & Bain, C. D. (2019). Fabrication of monolayers of uniform polymeric particles by inkjet printing of monodisperse emulsions produced by microfluidics. Lab on a Chip, 19(18), 3077-3085. https://doi.org/10.1039/c9lc00588a
- Drying of ethanol/water droplets containing silica nanoparticlesShi, J., Yang, L., & Bain, C. D. (2019). Drying of ethanol/water droplets containing silica nanoparticles. ACS Applied Materials and Interfaces, 11(15), 14275-14285. https://doi.org/10.1021/acsami.8b21731
- Drop-on-demand satellite-free drop formation for precision fluid deliveryYang, L., Kapur, N., Wang, Y., Fiesser, F., Bierbrauer, F., Wilson, M. C., Sabey, T., & Bain, C. D. (2018). Drop-on-demand satellite-free drop formation for precision fluid delivery. Chemical Engineering Science, 186, 102-115. https://doi.org/10.1016/j.ces.2018.04.014
- Effect of Surface Freezing on Stability of Oil-in-Water EmulsionsTokiwa, Y., Sakamoto, H., Takiue, T., Aratono, M., Matsubara, H., & Bain, C. D. (2018). Effect of Surface Freezing on Stability of Oil-in-Water Emulsions. Langmuir, 34(21), 6205-6209. https://doi.org/10.1021/acs.langmuir.8b01088
- Combining Inkjet Printing with Emulsion Solvent Evaporation to Pattern Polymeric ParticlesDeng, R., Yang, L., & Bain, C. D. (2018). Combining Inkjet Printing with Emulsion Solvent Evaporation to Pattern Polymeric Particles. ACS Applied Materials and Interfaces, 10(15), 12317-12322. https://doi.org/10.1021/acsami.8b02017
- Ink-Jet Printing of High-Molecular-Weight Polymers in Oil-in-Water EmulsionsJohns, A. S., & Bain, C. D. (2017). Ink-Jet Printing of High-Molecular-Weight Polymers in Oil-in-Water Emulsions. ACS Applied Materials and Interfaces, 9(27), 22918-22926. https://doi.org/10.1021/acsami.7b04454
- Microemulsion Droplets in Optical TrapsHargreaves, A., Gregson, F., Kirby, A., Engelskirchen, A., & Bain, C. (2015). Microemulsion Droplets in Optical Traps. Journal of Molecular Liquids, 210(A), 9-19. https://doi.org/10.1016/j.molliq.2015.06.028
- Time-resolved phase-sensitive second harmonic generation spectroscopyNowakowski, P. J., Woods, D. A., Bain, C. D., & Verlet, J. R. (2015). Time-resolved phase-sensitive second harmonic generation spectroscopy. Journal of Chemical Physics, 142(8). https://doi.org/10.1063/1.4909522
- Printing Small Dots from Large DropsTalbot, E. L., Yow, H. N., Yang, L., Berson, A., Biggs, S. R., & Bain, C. D. (2015). Printing Small Dots from Large Drops. ACS Applied Materials & Interfaces, 7(6), 3782-3790. https://doi.org/10.1021/am5087177
- Control of the Particle Distribution in Inkjet Printing through an Evaporation-Driven Sol-Gel TransitionTalbot, E., Yang, L., Berson, A., & Bain, C. (2014). Control of the Particle Distribution in Inkjet Printing through an Evaporation-Driven Sol-Gel Transition. ACS Applied Materials and Interfaces, 6(12), 9572-9583. https://doi.org/10.1021/am501966n
- Determination of dynamic surface tension and viscosity of non-Newtonian fluids from drop oscillationsYang, L., Kazmierski, B. K., Hoath, S. D., Jung, S., Hsiao, W., Wang, Y., Berson, A., Harlen, O., Kapur, N., & Bain, C. D. (2014). Determination of dynamic surface tension and viscosity of non-Newtonian fluids from drop oscillations. Physics of Fluids, 26(11), Article 113103. https://doi.org/10.1063/1.4901823
- A new pathway for the re-equilibration of micellar surfactant solutions.Griffiths, I., Breward, C., Colegate, D., Dellar, P., Howell, P., & Bain, C. (2013). A new pathway for the re-equilibration of micellar surfactant solutions. Soft Matter, 9(3), 853-863. https://doi.org/10.1039/c2sm27154k
- A Maxwell-Stefan-Derjaguin-Grahame model of the concentration profile of a charged solute in the polarisation layer.Vasan, S., Bain, C., Field, R., & Cui, Z. (2006). A Maxwell-Stefan-Derjaguin-Grahame model of the concentration profile of a charged solute in the polarisation layer. Desalination, 200(1-3), 175-177. https://doi.org/10.1016/j.desal.2006.03.286
- Optical sculpture: controlled deformation of emulsion droplets with ultralow interfacial tensions using optical tweezersWard, A., Berry, M., Mellor, C., & Bain, C. (2006). Optical sculpture: controlled deformation of emulsion droplets with ultralow interfacial tensions using optical tweezers. Chemical Communications, 43, 4515-4517. https://doi.org/10.1039/b610060k
- Polarization effects in optically bound particle arraysMellor, C., Fennerty, T., & Bain, C. (2006). Polarization effects in optically bound particle arrays. Optics Express, 14(21), 10079-10088. https://doi.org/10.1364/oe.14.010079

