Staff profile

| Affiliation | Telephone |
|---|---|
| Director of Education in the Department of Physics | +44 (0) 191 33 43648 |
| Associate Professor in the Department of Physics | +44 (0) 191 33 43648 |
Biography
Responsibilities
I am Director of Education in the Department of Physics and have overall responsibility for educational policy and delivery of teaching within the Department. This role includes Chairing the Department's Education Committee and membership of: Physics Board of Studies, Senior Management Committee, Departmental Progression Committee, Board of Examiners, Research Staff Consultative Committee and Faculty Education Committee.
I have a passion for curriculum development, adapting the learning environment in response to technological developments (such as generative Artificial Intelligence) and ensuring the delivery of an excellent learning experience in consultation with both students and colleagues.
Research
I am a member of the Condensed Matter Physics section (https://www.dur.ac.uk/research/institutes-and-centres/condensed-matter-physics/) and lead a small research group focused on nanostructured materials, with particular emphasis on sustainability, energy conversion and energy storage.
Energy Conversion and Storage
Low-cost, environmentally sustainable, and safe electrochemical energy storage and conversion is crucial for the green energy transition. Our work addresses two areas:
(1) Supercapacitors
Current battery technologies face limitations including short cycle life, environmental impact, safety concerns, and recycling difficulties. Supercapacitors address many of these issues but suffer from low energy density, which must be improved for broader adoption. By the use of low concentrations of two-dimensional materials such as few layer graphene, produced through environmentally sustainable shear exfoliation into aqueous suspensions, as a conductive additive in activated carbon electrodes we have demonstrated that significant improvements in rate capability and energy storage are possible in electric double layer capacitors.

Scanning Electron Micrographs of few-layer graphene infused activated carbon electrodes.
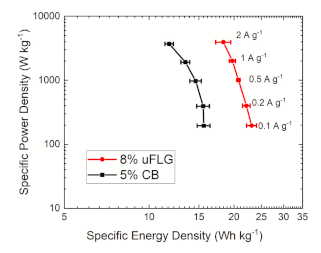
Ragone plot demonstrating the increase in electrochemical energy storage performance of activated electrodes containing 8% by weight few layer graphene compared with 5% by weight carbon black (the optimised concentration for each conductive additive).
See: Williams, R. E., Sukumaran, S., Abbas, Q., & Hunt, M. R. C. (2024). Few-layer graphene as an ‘active’ conductive additive for flexible aqueous supercapacitor electrodes. Carbon, 218, 118744. https://doi.org/10.1016/j.carbon.2023.118744
The matching of electrode material and electrolyte is a key aspect of optimising performance, including longevity in supercapacitors. By considering the behaviour of nanostructured binder-free supercapacitor electrodes consisting of layered MoS2 supported on carbon cloth in six aqueous electrolytes and correlating the results with morphology and composition we were able to identify the electrolyte which offered the best combination of performance and cycle life. The value of this approach was demonstrated by the production of an asymmetric supercapacitor consisting of binder free carbon cloth supported MoS2 and MnO2 electrodes in 1.0M Na2SO4 with a working voltage window of 2.0 V and a capacity retention of 80% after 15,000 charge-discharge cycles.
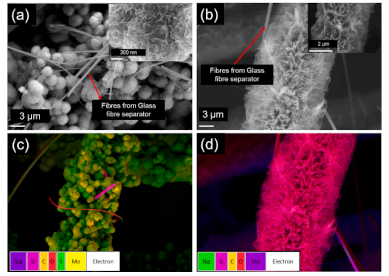
Analysis of electrodes after 15000 charge/discharge cycles in a two-terminal asymmetric supercapacitor: (a) SEM image of MoS2@CC (the inset is a high-resolution image of the same material); (b) SEM image of MnO2@CC; Element mapped images of: (c) MoS2@CC and (d) MnO2@CC.
See: Faisal, M. M., Rigby, O. M., Campbell, S., & Hunt, M. R. C. (2025). Optimised electrochemical energy storage performance of MoS2 anchored to carbon cloth for advanced asymmetric aqueous supercapacitors. Electrochimica Acta, 532, 146294. https://doi.org/10.1016/j.electacta.2025.146294
(2) Energy Conversion
The high gravimetric energy density of H2, the high Earth-abundance of potential sources and the fact that water, rather than environmentally-damaging gases, are the byproduct of its combustion make it highly attractive as a carbon-neutral energy source, if produced by 'green' routes. Electrolysis of water employing renewable energy sources is such a route but suffers from the high cost of electrocatalysts typically employed in this process. Non-noble metal bases electrocatalysts such as supported nanostructures MoS2 offer practical and scalable alternatives. In recent work we have demonstrated that a careful balance between the electrolyte accessible surface area, resulting from its hierarchical nanostructured morphology, concentration of active sites, proportion of the conductive electroactive metastable 1T phase of MoS2 compared with the stable 2H phase and crystallinity is required to achieve the best catalytic behaviour.
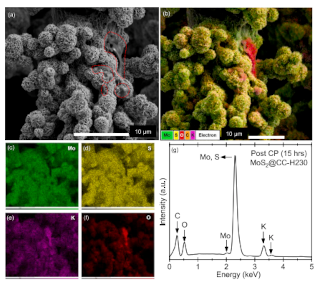
Post HER characterisation of optimised MoS2@CC: (a) SEM micrograph indicating the retention of hierarchical structure after prolonged hydrogen generation. (b-f) Elementally mapped SEM demonstrating that oxygen within the electrocatalyst after cycling can be associated with residual KOH from the electrolyte. (g) EDS point spectrum indicating the presence of elements originating only from the catalyst and electrolyte.
See: Faisal, M. M., Šiller, L., & Hunt, M. R. C. (2025). Engineering composition and structure of binder-free MoS2 anchored to carbon cloth for enhanced hydrogen evolution performance. Electrochimica Acta, 146809. https://doi.org/10.1016/j.electacta.2025.146809
Non-Equilibrium Self-Organised Nanoscale Surface Structures
The competition between roughening induced by the ion induced irradiation of solid surfaces and relaxation processes such as diffusion which smooth the surface can lead to the formation of quasi-periodic structures such as dots, holes and ripples. This process provides a maskless route for the production of functional surfaces and templates which can be used in applications as diverse as solar cells and bio-compatible surfaces. In our research we combine experimental measurement with numerical simulation to understand these processes with the ultimate goal of deriving predictive rules for the production of surface patterns tailored to specific uses.
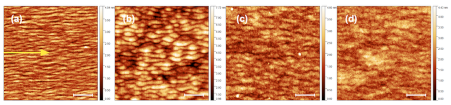
AFM topographs of pattern formation on Si(111) due to Ar+ ion bombardment (Eion = 3.8 keV, ion flux f = 1.6×1013 ions cm-2 s-1, incident angle θ = 60º, at room temperature) as a function of fluence: (a) 1.7×1017 ions cm-2, (b) 2.3×1017 ions cm-2, (c) 3.5×1017 ions cm-2 and (d) 4.6×1017 ions cm-2. The scale bar in each image is 400 nm in length. The projection of the ion beam onto the target surface is indicated by the yellow arrow in panel (a).
See: Mekki, M. B., & Hunt, M. R. C. (2024). Linear vs. Non-linear Behaviour in Ion Irradiation Nanostructuring of Nickel and Silicon Surfaces. Journal of Physics: Conference Series, 2751(1), 012001. https://doi.org/10.1088/1742-6596/2751/1/012001
Liquid-liquid interface deposition of two-dimensional materials
Thin films and van der Waals heterostructures (vdWHs) derived from two-dimensional solids offer enormous potential for a broad range of novel, energy efficient devices, however their use is currently hampered by slow, labour-intensive fabrication methods often employing hazardous chemicals. We have demonstrated a novel liquid-liquid interface technique for rapid, low-cost, and environmentally-friendly production of ultra-thin films and vdWHs of two-dimensional solids from aqueous surfactant-stabilized suspensions. The approach is generic to two-dimensional materials which can be stabilized in aqueous suspension by a surfactant and the resulting films can be transferred to an arbitrary substrate by a range of approaches. We have demonstrated the wide applicability of this technique through production of thin films on a variety of substrates, deposition of transparent, highly conductive graphene films by the fabrication of a vdWHs of MoS2, WS2 and few-layer graphene.
Current Teaching
Undergraduate:
- PHYS2641 Laboratory Skills and Electronics: Bridge Projects
- PHYS3701 BSc Projects
- PHYS4213 MPhys Projects
Postgraduate
- Symmetry and Group Theory in Physics and Chemistry
Previous Teaching Leadership and Administration
- Deputy Chair Physics Board of Examiners (2023-2025)
- Chair Physics AI Working Group (2023-2025)
- Level 1 Course Director (2019-2025)
- Level 3 Laboratory Leader (2012-2017)
- Member of Laboratories Committee (2012-2017)
- Chair Level 3 Examination Papers Committee (2015-2017)
- Member of Level 2 Examination Paper Committee (2014)
- Undergraduate Admissions Selector (2015-2017)
Publications
Book review
- An insidious and ubiquitous menaceHunt, M., & Siller, L. (2015). An insidious and ubiquitous menace. Physics World., 28(9), 40-41. https://doi.org/10.1088/2058-7058/28/9/38
Chapter in book
- Na-Ion BatteriesMirzaeian, M., Abbas, Q., Hunt, M. R., Galeyeva, A., & Raza, R. (2021). Na-Ion Batteries. In Reference Module in Materials Science and Materials Engineering. Elsevier. https://doi.org/10.1016/b978-0-12-815732-9.00052-8
- Materials for Sodium-Ion BatteriesAbbas, Q., Mirzaeian, M., & Hunt, M. R. (2020). Materials for Sodium-Ion Batteries. In Reference Module in Materials Science and Materials Engineering. https://doi.org/10.1016/b978-0-12-803581-8.12115-0
- Ionic Liquids BatteriesAbbas, Q., & Hunt, M. R. (2020). Ionic Liquids Batteries. In Reference module in materials science and materials engineering. Elsevier. https://doi.org/10.1016/b978-0-12-815732-9.00035-8
- Carbon Onions.Butenko, Y., Šiller, L., & Hunt, M. R. (2014). Carbon Onions. In Y. Gogotsi & V. Presser (Eds.), Carbon Nanomaterials. (pp. 279-302). CRC Press. https://doi.org/10.1201/b15591-11
- Carbon Onions.Butenko, Y. V., Šiller, L., & Hunt, M. (2010). Carbon Onions. In K. Sattler (Ed.), Handbook of Nanophysics: Clusters and Fullerenes (pp. 1-18). CRC Press. https://doi.org/10.1201/9781420075557-39
Conference Paper
- Linear vs. Non-linear Behaviour in Ion Irradiation Nanostructuring of Nickel and Silicon SurfacesMekki, M. B., & Hunt, M. R. C. (2024). Linear vs. Non-linear Behaviour in Ion Irradiation Nanostructuring of Nickel and Silicon Surfaces. Journal of Physics: Conference Series, 2751(1), Article 012001. https://doi.org/10.1088/1742-6596/2751/1/012001
- Few-Layer Graphene As an Electrode, Electrode Additive and an Interfacial Layer in Aqueous SupercapacitorsWilliams, R., Sukumaran, S., Abbas, Q., & Hunt, M. (2022, October 9). Few-Layer Graphene As an Electrode, Electrode Additive and an Interfacial Layer in Aqueous Supercapacitors. https://doi.org/10.1149/ma2022-028660mtgabs
Journal Article
- Engineering composition and structure of binder-free MoS2 anchored to carbon cloth for enhanced hydrogen evolution performanceFaisal, M., Šiller, L., & Hunt, M. R. (2025). Engineering composition and structure of binder-free MoS2 anchored to carbon cloth for enhanced hydrogen evolution performance. Electrochimica Acta, Article 146809. https://doi.org/10.1016/j.electacta.2025.146809
- Optimised electrochemical energy storage performance of MoS2 anchored to carbon cloth for advanced asymmetric aqueous supercapacitorsFaisal, M. M., Rigby, O. M., Campbell, S., & Hunt, M. R. C. (2025). Optimised electrochemical energy storage performance of MoS2 anchored to carbon cloth for advanced asymmetric aqueous supercapacitors. Electrochimica Acta, 532, Article 146294. https://doi.org/10.1016/j.electacta.2025.146294
- Synthesis and characterization of ammonia strengthened and ambient dried N-doped hydrophilic graphene aerogel with good electrical conductivityXiong, F., Wang, J., Dickman, N., Liu, Y., Hunt, M. R., & Šiller, L. (2025). Synthesis and characterization of ammonia strengthened and ambient dried N-doped hydrophilic graphene aerogel with good electrical conductivity. Vacuum, 232, Article 113846. https://doi.org/10.1016/j.vacuum.2024.113846
- Few-layer graphene as an ‘active’ conductive additive for flexible aqueous supercapacitor electrodesWilliams, R., Sukumaran, S., Abbas, Q., & Hunt, M. (2023). Few-layer graphene as an ‘active’ conductive additive for flexible aqueous supercapacitor electrodes. Carbon, 218, Article 118744. https://doi.org/10.1016/j.carbon.2023.118744
- Current State and Future Prospects for Electrochemical Energy Storage and Conversion SystemsAbbas, Q., Mirzaeian, M., Hunt, M. R., Hall, P., & Raza, R. (2020). Current State and Future Prospects for Electrochemical Energy Storage and Conversion Systems. Energies, 13(21), Article 5847. https://doi.org/10.3390/en13215847
- Pseudocapacitive Effect of Carbons Doped with Different Functional Groups as Electrode Materials for Electrochemical CapacitorsMirzaeian, M., Abbas, Q., Hunt, M. R., & Hall, P. (2020). Pseudocapacitive Effect of Carbons Doped with Different Functional Groups as Electrode Materials for Electrochemical Capacitors. Energies, 13(21), Article 5577. https://doi.org/10.3390/en13215577
- Electron Compton scattering and the measurement of electron momentum distributions in solidsTalmantaite, A., Hunt, M., & Mendis, B. (2020). Electron Compton scattering and the measurement of electron momentum distributions in solids. Journal of Microscopy, 279(3), 185-188. https://doi.org/10.1111/jmi.12854
- Morphology control of nickel nanoparticles prepared in situ within silica aerogels produced by novel ambient pressure dryingLu, J., Wang, J., Hassan, K. T., Talmantaite, A., Xiao, Z., Hunt, M. R., & Šiller, L. (2020). Morphology control of nickel nanoparticles prepared in situ within silica aerogels produced by novel ambient pressure drying. Scientific Reports, 10(1), Article 11743. https://doi.org/10.1038/s41598-020-68510-4
- Bioinspired Synthesis of Monolithic and Layered AerogelsHan, X., Hassan, K. T., Harvey, A., Kulijer, D., Oila, A., Hunt, M. R., & Siller, L. (2018). Bioinspired Synthesis of Monolithic and Layered Aerogels. Advanced Materials, 30(23), Article 1706294. https://doi.org/10.1002/adma.201706294
- Synthesis and Characterisation of Reduced Graphene Oxide/Bismuth Composite for Electrodes in Electrochemical Energy Storage DevicesWang, J., Zhang, H., Hunt, M., Charles, A., Tang, J., Bretcanu, O., Walker, D., Hassan, K., Sun, Y., & Siller, L. (2017). Synthesis and Characterisation of Reduced Graphene Oxide/Bismuth Composite for Electrodes in Electrochemical Energy Storage Devices. ChemSusChem, 10(2), 363-371. https://doi.org/10.1002/cssc.201601553
- Sulphur-Containing Compounds as a Response in Sea Urchins Exposed to Alkylated Silicon Nanocrystals and SiO2-Coated Iron Oxide NanoparticlesSiller, L., Piticharoenphun, S., Lemloh, M., Horrocks, B., Kaulich, B., Gianoncelli, A., Hunt, M., Brummer, F., & Medakovic, D. (2016). Sulphur-Containing Compounds as a Response in Sea Urchins Exposed to Alkylated Silicon Nanocrystals and SiO2-Coated Iron Oxide Nanoparticles. Key Engineering Materials, 672, 312-327. https://doi.org/10.4028/www.scientific.net/kem.672.312
- Facile technique for the removal of metal contamination from grapheneWells, G., Hunt, M., Hopf, T., Vassilevski, K., Escobedo-Cousin, E., Horsfall, A., Goss, J., & O’Neill, A. (2015). Facile technique for the removal of metal contamination from graphene. Journal of Vacuum Science and Technology B, 33(5), Article 051802. https://doi.org/10.1116/1.4928422
- Electrical characterization of epitaxial graphene field-effect transistors with high-k Al2O3 gate dieletric fabricated on SiC substratesHopf, T., Vassilevski, K., Escobedo-Cousin, E., King, P., Wright, N., O’Neill, A., Horsfall, A., Goss, J., Wells, G., & Hunt, M. (2015). Electrical characterization of epitaxial graphene field-effect transistors with high-k Al2O3 gate dieletric fabricated on SiC substrates. Materials Science Forum, 821-823, 937-940. https://doi.org/10.4028/www.scientific.net/msf.821-823.937
- Determination of the adhesion energy of graphene on SiC(0001) via measurement of pleat defectsWells, G., Hopf, T., Vassilevski, K., Escobedo-Cousin, E., Wright, N., Horsfall, A., Goss, J., O’Neill, A., & Hunt, M. (2014). Determination of the adhesion energy of graphene on SiC(0001) via measurement of pleat defects. Applied Physics Letters, 105(19), Article 193109. https://doi.org/10.1063/1.4901941
- Dirac point and transconductance of top-gated graphene field-effect transistors operating at elevated temperatureHopf, T., Vassilevski, K., Escobedo-Cousin, E., King, P., Wright, N., O’Neill, A., Horsfall, A., Goss, J., Wells, G., & Hunt, M. (2014). Dirac point and transconductance of top-gated graphene field-effect transistors operating at elevated temperature. Journal of Applied Physics, 116(15), Article 154504. https://doi.org/10.1063/1.4898562
- Optimizing the Vacuum Growth of Epitaxial Graphene on 6H-SiCHopf, T., Vassilevski, K., Escobedo-Cousin, E., Wright, N., O’Neill, A. G., Horsfall, A., Goss, J. P., Barlow, A., Wells, G., & Hunt, M. (2014). Optimizing the Vacuum Growth of Epitaxial Graphene on 6H-SiC. Materials Science Forum, 778-780, 1154-1157. https://doi.org/10.4028/www.scientific.net/msf.778-780.1154
- In situ formation of onion-like carbon from the evaporation of ultra-dispersed nanodiamondsKrishnamurthy, S., Butenko, Y. V., Dhanak, V., Hunt, M., & Siller, L. (2013). In situ formation of onion-like carbon from the evaporation of ultra-dispersed nanodiamonds. Carbon, 52, 145-149. https://doi.org/10.1016/j.carbon.2012.09.015
- Extraordinarily Long-Ranged Structural Relaxation in Defective Achiral Carbon NanotubesHunt, M., & Clark, S. (2012). Extraordinarily Long-Ranged Structural Relaxation in Defective Achiral Carbon Nanotubes. Physical Review Letters, 109(26), Article 265502. https://doi.org/10.1103/physrevlett.109.265502
- DNA-modified silicon nanocrystals studied by X-ray luminescence and X-ray absorption spectroscopies: Observation of a strong infra-red luminescence bandCoxon, P., Newman, M., Hunt, M., O’Farrell, N., Horrocks, B., Poolton, N., & Siller, L. (2012). DNA-modified silicon nanocrystals studied by X-ray luminescence and X-ray absorption spectroscopies: Observation of a strong infra-red luminescence band. Journal of Applied Physics, 111(5), Article 054311. https://doi.org/10.1063/1.3691600
- Surface Hubbard U of alkali fulleridesMacovez, R., Hunt, M., Goldoni, A., Pedio, M., & Rudolf, P. (2011). Surface Hubbard U of alkali fullerides. Journal of Electron Spectroscopy and Related Phenomena, 183(1-3), 94-100. https://doi.org/10.1016/j.elspec.2010.07.006
- Core and valence exciton formation in x-ray absorption, x-ray emission and x-ray excited optical luminescence from passivated Si nanocrystals at the Si L-2,L-3 edgeSiller, L., Krishnamurthy, S., Kjeldgaard, L., Horrocks, B., Chao, Y., Houlton, A., Chakraborty, A., & Hunt, M. (2009). Core and valence exciton formation in x-ray absorption, x-ray emission and x-ray excited optical luminescence from passivated Si nanocrystals at the Si L-2,L-3 edge. Journal of Physics: Condensed Matter, 21(9), Article 095005. https://doi.org/10.1088/0953-8984/21/9/095005
- Metal-to-insulator transition in thin-film polymeric AC(60)Macovez, R., Hunt, M., Shan, J., Goldoni, A., Pichler, T., Pedio, M., Moras, P., Castellarin-Cudia, C., Schiessling, J., Venema, L., & Rudolf, P. (2009). Metal-to-insulator transition in thin-film polymeric AC(60). New Journal of Physics, 11(2), Article 023035. https://doi.org/10.1088/1367-2630/11/2/023035
- Gold Nitride: Preparation and PropertiesSiller, L., Alves, L., Brieva, A., Butenko, Y., & Hunt, M. (2009). Gold Nitride: Preparation and Properties. Topics in Catalysis, 52(11), 1604-1610. https://doi.org/10.1007/s11244-009-9281-6
- Surface and bulk components in angle-resolved core-level photoemission spectroscopy of graphiteHunt, M. R. (2008). Surface and bulk components in angle-resolved core-level photoemission spectroscopy of graphite. Physical Review B, 78(15), Article 153408. https://doi.org/10.1103/physrevb.78.153408
- Potassium intercalation of carbon onions 'opened' by carbon dioxide treatmentButenko, Y. V., Chakraborty, A., Peltekis, N., Krishnamurthy, S., Dhanak, V., Hunt, M., & Siller, L. (2008). Potassium intercalation of carbon onions ’opened’ by carbon dioxide treatment. Carbon, 46(8), 1133-1140. https://doi.org/10.1016/j.carbon.2008.04.012
- A photoelectron spectroscopy study of ion-irradiation induced defects in single-wall carbon nanotubesChakraborty, A., Wooley, R., Butenko, Y. V., Siller, L., & Hunt, M. (2007). A photoelectron spectroscopy study of ion-irradiation induced defects in single-wall carbon nanotubes. Carbon, 45, 2744-2750. https://doi.org/10.1016/j.carbon.2007.09.036
- Influence of star-like iridium complexes in the graphoepitaxy of polyfluorene thin films.Knaapila, M., Torkkeli, M., Lyons, B., Hunt, M., Hase, T., Seeck, O., Bouchenoire, L., Serimaa, R., & Monkman, A. (2006). Influence of star-like iridium complexes in the graphoepitaxy of polyfluorene thin films. Physical Review B, 74, 1-5. https://doi.org/10.1103/physrevb.74.214203
- Chemical vapor deposition growth of carbon nanotubes on Si substrates using Fe catalyst: What happens at the nanotube/Fe/Si interface.Chakraborty, A. K., Jacobs, J., Anderson, C., Roberts, C., & Hunt, M. R. (2006). Chemical vapor deposition growth of carbon nanotubes on Si substrates using Fe catalyst: What happens at the nanotube/Fe/Si interface. Journal of Applied Physics, 100(8), 1-6. https://doi.org/10.1063/1.2360774
- Optical luminescence from alkyl-passivated Si nanocrystals under vacuum ultraviolet excitation: Origin and temperature dependence of the blue and orange emissions.Chao, Y., Houlton, A., Horrocks, B., Hunt, M., Poolton, N., Yang, J., & Siller, L. (2006). Optical luminescence from alkyl-passivated Si nanocrystals under vacuum ultraviolet excitation: Origin and temperature dependence of the blue and orange emissions. Applied Physics Letters, 88(26), 1-3. https://doi.org/10.1063/1.2216911
- Rapid thermal oxidation of Ge-rich Si1-xGex heterolayersBera, M., Chakraborty, S., Das, R., Dalapati, G., Chattopadhyay, S., Samanta, S., Yoo, W., Chakraborty, A., Butenko, Y., Siller, L., Hunt, M., Saha, S., & Maiti, C. (2006). Rapid thermal oxidation of Ge-rich Si1-xGex heterolayers. Journal of Vacuum Science and Technology A, 24(1), 84-90.
- Reactions and luminescence in passivated Si nanocrystallites induced by vacuum ultraviolet and soft-x-ray photonsChao, Y., Krishnamurthy, S., Montalti, M., Lie, L., Houlton, A., Horrocks, B., Kjeldgaard, L., Dhanak, V., Hunt, M., & Šiller, L. (2005). Reactions and luminescence in passivated Si nanocrystallites induced by vacuum ultraviolet and soft-x-ray photons. Journal of Applied Physics, 98(4), Article 044316. https://doi.org/10.1063/1.2012511
- Gold film with gold nitride-A conductor but harder than goldŠiller, L., Peltekis, N., Krishnamurthy, S., Chao, Y., Bull, S., & Hunt, M. (2005). Gold film with gold nitride-A conductor but harder than gold. Applied Physics Letters, 86(22). https://doi.org/10.1063/1.1941471
- Photoemission study of onionlike carbons produced by annealing nanodiamondsButenko, Y. V., Krishnamurthy, S., Chakraborty, A., Kuznetsov, V., Dhanak, V., Hunt, M., & Šiller, L. (2005). Photoemission study of onionlike carbons produced by annealing nanodiamonds. Physical Review B, 71, 075420-.
- Nitrogen ion irradiation of Au(110) : Photoemission spectroscopy and possible crystal structures of gold nitrideKrishnamurthy, S., Montalti, M., Wardle, M., Shaw, M., Briddon, P., Svensson, K., Hunt, M., & Šiller, L. (2004). Nitrogen ion irradiation of Au(110) : Photoemission spectroscopy and possible crystal structures of gold nitride. Physical Review B, 70(4). https://doi.org/10.1103/physrevb.70.045414
- Photoemission spectroscopy of clean and potassium-intercalated carbon onionsMontalti, M., Krishnamurthy, S., Chao, Y., Butenko, Y., Kuznetsov, V., Dhanak, V., Hunt, M., & Šiller, L. (2003). Photoemission spectroscopy of clean and potassium-intercalated carbon onions. Physical Review B, 67(11). https://doi.org/10.1103/physrevb.67.113401
- Adsorption and manipulation of endohedral and higher fullerenes on Si(100)-2×1Butcher, M., Nolan, J., Hunt, M., Beton, P., Dunsch, L., Kuran, P., Georgi, P., & Dennis, T. (2003). Adsorption and manipulation of endohedral and higher fullerenes on Si(100)-2×1. Physical Review B, 67, 125413-.
- Final-state interference effects in valence band photoemission of (C₅₉N)₂Hunt, M., Pichler, T., Šiller, L., Brühwiler, P., Golden, M., Tagmatarchis, N., Prassides, K., & Rudolf, P. (2002). Final-state interference effects in valence band photoemission of (C₅₉N)₂. Physical Review B, 66(19). https://doi.org/10.1103/physrevb.66.193404
- Nitrogen Ion Irradiation Of Au(110): Formation Of Gold NitrideSiller, L., Hunt, M., Brown, J., Coquel, J., & Rudolf, P. (2002). Nitrogen Ion Irradiation Of Au(110): Formation Of Gold Nitride. Surface Science, 513, 78-82. https://doi.org/10.1016/s0039-6028%2802%2901150-0
- Thermally induced decomposition of single-wall carbon nanotubes adsorbed on H/Si(111)Hunt, M. R., Montalti, M., Chao, Y., Krishnamurthy, S., Dhanak, V. R., & Šiller, L. (2002). Thermally induced decomposition of single-wall carbon nanotubes adsorbed on H/Si(111). Applied Physics Letters, 81(25), 4847-4849. https://doi.org/10.1063/1.1530747
- Photoemission spectroscopy of the evolution of In-terminated InP(100)-(2×4) as a function of temperature: Surface- and cluster-related In 4d linesChao, Y., Svensson, K., Radosavkić, D., Dhanak, V., Hunt, M., & Šiller, L. (2002). Photoemission spectroscopy of the evolution of In-terminated InP(100)-(2×4) as a function of temperature: Surface- and cluster-related In 4d lines. Physical Review B, 66(7), Article 075323. https://doi.org/10.1103/physrevb.66.075323
- Orientationally ordered island growth of higher fullerenes on Ag/Si(111)-(3×3)R30°Butcher, M., Nolan, J., Hunt, M., Beton, P., Dunsch, L., Kuran, P., Georgi, P., & Dennis, T. (2001). Orientationally ordered island growth of higher fullerenes on Ag/Si(111)-(3×3)R30°. Physical Review B, 64(19), Article 195401. https://doi.org/10.1103/physrevb.64.195401
- Photoemission study of chemisorption of C₆₀ on InP(100)Chao, Y., Svensson, K., Radosavkić, D., Dhanak, V., Šiller, L., & Hunt, M. (2001). Photoemission study of chemisorption of C₆₀ on InP(100). Physical Review B, 64(23), Article 235331. https://doi.org/10.1103/physrevb.64.235331
- Proof for trivalent Sc ions in Sc₂\@C₈₄ from high-energy spectroscopyPichler, T., Hu, Z., Grazioli, C., Legner, S., Knupfer, M., Golden, M., Fink, J., de Groot, F., Hunt, M., Rudolf, P., Follath, R., Jung, C., Kjeldgaard, L., Brühwiler, P., Inakuma, M., & Shinohara, H. (2000). Proof for trivalent Sc ions in Sc₂\@C₈₄ from high-energy spectroscopy. Physical Review B, 62(19), 13196-13201. https://doi.org/10.1103/physrevb.62.13196
- Electron-beam-induced fragmentation in ultrathin C₆₀ films on Si(100)-2×1-H: Mechanisms of cage destructionHunt, M. R., Schmidt, J., & Palmer, R. E. (1999). Electron-beam-induced fragmentation in ultrathin C₆₀ films on Si(100)-2×1-H: Mechanisms of cage destruction. Physical Review B, 60(8), 5927-5937. https://doi.org/10.1103/physrevb.60.5927
- The development of metallic behaviour in clusters on surfacesHunt, M., & Palmer, R. (1998). The development of metallic behaviour in clusters on surfaces. "Philosophical Transactions A: Mathematical, Physical and Engineering Sciences", 356, 231-.
- Electron-beam damage of C₆₀ films on hydrogen-passivated Si(100)Hunt, M. R., Schmidt, J., & Palmer, R. E. (1998). Electron-beam damage of C₆₀ films on hydrogen-passivated Si(100). Applied Physics Letters, 72, 323-325. https://doi.org/10.1063/1.120725
- Electron-stimulated reaction of C₆₀ with a model etching gas, SF₆Coquel, J., Hunt, M. R., Siller, L., Palmer, & Richard, E. (1998). Electron-stimulated reaction of C₆₀ with a model etching gas, SF₆. Journal of Applied Physics, 84, 4603-4610.
- Localization of substrate-induced modification in the electronic structureof C₆₀sat fullerene-metal interfacesHunt, M. R., Rudolf, P., & Modesti, S. (1997). Localization of substrate-induced modification in the electronic structureof C₆₀sat fullerene-metal interfaces. Physical Review B, 55, 7882-7888. https://doi.org/10.1103/physrevb.55.7882
- Photoemission and electron-energy-loss-spectroscopy study of C₆₀ monolayers adsorbed on Cs-precovered Au(110) and of bulk distilled CsxC₆₀Hunt, M. R., Rudolf, P., & Modesti, S. (1997). Photoemission and electron-energy-loss-spectroscopy study of C₆₀ monolayers adsorbed on Cs-precovered Au(110) and of bulk distilled Cs<SUB>x</SUB>C₆₀. Physical Review B, 55, 7889-7903. https://doi.org/10.1103/physrevb.55.7889
- Film growth and surface reactions of C₆₀ on Si(100)H(2×1)Schmidt, J., Hunt, M., Miao, P., & Palmer, R. (1997). Film growth and surface reactions of C₆₀ on Si(100)H(2×1). Physical Review B, 56, 9918-9924. https://doi.org/10.1103/physrevb.56.9918
- Electronic And Geometric Structure Of Cs On Graphite (0001)Hunt, M., Durston, P., & Palmer, R. (1996). Electronic And Geometric Structure Of Cs On Graphite (0001). Surface Science, 364, 266-272. https://doi.org/10.1016/0039-6028%2896%2900629-2
- The Structural And Vibrational Properties Of C-60 Adsorbed On The Graphite(0001) SurfaceHunt, M., & Palmer, R. (1996). The Structural And Vibrational Properties Of C-60 Adsorbed On The Graphite(0001) Surface. Surface Review and Letters, 3, 937-942.
- Temperature Dependence Of The Electronic And Vibrational Excitations Of C-60 Adsorbed On Si(100)-2x1Hunt, M. (1996). Temperature Dependence Of The Electronic And Vibrational Excitations Of C-60 Adsorbed On Si(100)-2x1. Journal of Physics: Condensed Matter, 8, L229-L235. https://doi.org/10.1088/0953-8984/8/14/002
- Growth And Electronic Excitations Of Cs/c-60 Layered Structures On GraphiteLi, Z., Hunt, M., & Palmer, R. (1996). Growth And Electronic Excitations Of Cs/c-60 Layered Structures On Graphite. Surface Science, 352, 442-446. https://doi.org/10.1016/0039-6028%2895%2901177-3
- Charge transfer and structure in C₆₀ adsorption on metal surfacesHunt, M., Modesti, S., Rudolf, P., & Palmer, R. (1995). Charge transfer and structure in C₆₀ adsorption on metal surfaces. Physical Review B, 51, 10039-10047. https://doi.org/10.1103/physrevb.51.10039
- New Resonances In High-resolution Eels Of Adsorbed Molecules - Electronic Excitation Of Physisorbed O-2Barnard, J., Hock, K., Siller, L., Hunt, M., Wendelken, J., & Palmer, R. (1993). New Resonances In High-resolution Eels Of Adsorbed Molecules - Electronic Excitation Of Physisorbed O-2. Surface Science, 291, 139-144. https://doi.org/10.1016/0039-6028%2893%2991485-8

